Thermodynamics and Kinetics
Professor Xiao-Qing Zhu has studied the thermodynamics and kinetics of various chemical reactions, especially, hydride transfer reactions for more than 30 years. In thermodynamic study, he developed a high-accurate experimental method to measure thermodynamic hydricities of various metal-free hydrides and by which the thermodynamic hydricities of more than 2000 metal-free hydrides in acetonitrile have been determined systematically. He developed an efficient thermodynamic platform to directly diagnose the mechanism of various hydride transfer reactions and by which the mechanisms of more than 25 ten thousand important hydride transfer reactions in acetonitrile have been clarified clearly. In kinetic study, he discovered the fundamental error of Marcus theory and established a new kinetic equation (Zhu equation) to replace Marcus equation. Zhu equation has accurately predicted the rate constants of 34782 hydride-, 11772 hydrogen atom- 39402 proton-, and more than 40000 electron-transfer reactions and 22477 reactions of Lewis bases with Lewis acids in acetonitrile at 298 K only according to one related thermo-kinetic parameter of each reactant, and no one is exception. Zhu equation succeeded in realizing the centenary dream of chemists: the rate constant of chemical reaction can be predicted directly based on the parameters of the reactants. In addition, Professor Xiaoqing Zhu also developed a new KIE kinetic model to replace the traditional KIE kinetic model. This new KIE model can be used not only interpret uniformly, but also predict the KIE values of various chemical reactions.
Reactions:XL + Y → X + YL
(L = e, e2, H+, H, H-, Cl-, CN-, NO, NO+...)
Mechanism:XL + Y → [X...L...Y]≠ → X + YL
Zhu kinetic model: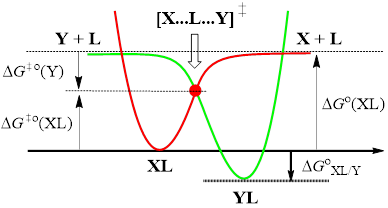
Zhu equation:DG≠XL/Y = ½(DG≠XL/X + DG≠YL/Y) + ½DGoXL/Y
or
DG≠XL/Y = DG≠o(XL) + DG≠o(Y)
DG≠o(XL) = ½DG≠XL/X + ½DGo(XL)
DG≠o(Y) = ½DG≠YL/Y - ½DGo(YL)

