Shoufei Zhu‘s Group: Iron-Catalyzed Regioselectivity-Controllable Hydrosilylation of Alkynes
As a cheap and easy-to-obtain transition metal, iron is abundant in the crust of earth, and has good biocompatibility. Therefore, the development of iron-catalyzed reactions has received increasing attention, and some important progress has been received. Nevertheless, due to the limited types of developed ligands applicable at this stage, the reported iron-based catalysts still have many problems in terms of reactivity, selectivity, types of substrates, functional group tolerance and stability. Therefore, most of them cannot match the rare-metal catalysts, and only have few practical applications. Moreover, because of the disadvantages of iron-based catalysts (such as poor stability, complex oxidation states & spin states), people have limited methods for characterizing the structure, and the research on the mechanism of iron-catalyzed reactions has been tardy - especially for the law of influence on the reactivity and selectivity affected by the spin state of open-shell iron-based catalysts, which has seriously restricted the development of iron-based catalysts and iron-catalyzed reactions. Thus, developing novel iron-based ligand framework, discovering new iron-catalyzed reactions and deeply exploring the reaction mechanism, especially revealing the law of influence of the spin states of iron-based catalysts on their catalytic performance, have important researching value.
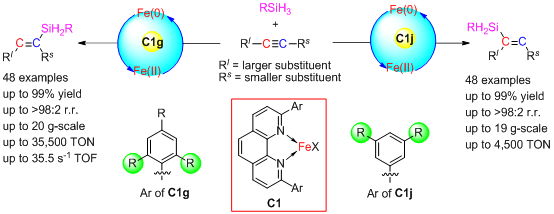
Figure 1. Iron-catalyzed regioselectivity-controllable hydrosilylation.
In the early stage, Shoufei Zhu (Professor and Dean of College of Chemistry, Nankai University) group firstly reported a transition metal-catalyzed reaction using substituted 2,9-diphenyl-1,10-phenanthroline as ligands, and found that complexes of iron with such ligands can efficiently catalyze the hydrosilylation of olefins and dihydrosilylation of alkynes, showing distinctive reactivity and selectivity (compared with former iron-based catalysts) (see: Nature Commun. 2018, 9, 221; J. Am. Chem. Soc. 2019, 141, 4579). Recently, Zhu group successfully used the developed iron-phenanthroline complexes to catalyze the hydrosilylation reaction of various terminal and internal alkynes, which showed high catalytic reactivity (conversion number: 35500; conversion frequency: 35.5/s), specific cis-addition selectivity, and excellent regioselectivity (Figure 1). More interestingly, the regioselectivity of the reactions can be completely reversed just by changing the aryl substituents on phenanthroline - for aromatic and aliphatic terminal alkynes, aromatic-alkyl and alkenyl-alkyl internal alkynes, and even asymmetrical dialkyl-substituted internal alkynes, when the aryl substituents are 2,4,6-trisubstituted aryls, the silyl groups will mainly add to the side of the substrates with less steric hindrance; relatively, when the aryl substituents of the phenanthroline are 3,5-disubstituted aryls, the silyl groups will mainly add to the side with larger steric hindrance. For the above reactions, the regioselectivity will be up to more than 98%, which is rarely reported in the former researches, and provides an excellent opportunity to understand the mechanism of iron-catalyzed reactions.
Based on this novel reaction, the di- or tri-substituted alkenylsilanes can be synthesized from easy-obtained alkyne substrates, with high efficiency and selectivity. Furthermore, combined with the easy-transforming property of carbon-silicon bonds, the research also provides a efficient method for the mass preparation of di- and tri-substituted alkenes under mild conditions, which significantly improves the synthesizing efficiency of a number of biological-active compounds (Figure 2A). Due to the double silicon-hydrogen bonds in the products, and silicon-containing polymers can be synthesized by hydrosilylation, this method may have further applications in the field of synthetic materials (Figure 2B).
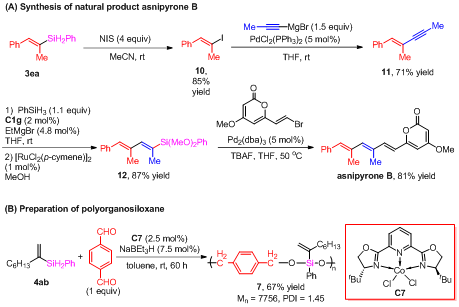
Figure 2. Examples of synthetic applications.
The isotope labeling experiments showed that the silicon and hydrogen added to the alkyne substrates are derived from the same silane, which indicates that the reaction is unlikely to undergo a redox process initiated by Fe-H or Fe-Si, and more likely to go through the 2e redox process (Figure 3A). Dynamic isotope experiments indicated that the hydrogen transfer process in the reaction is likely to be a decisive step (Figure 3B).
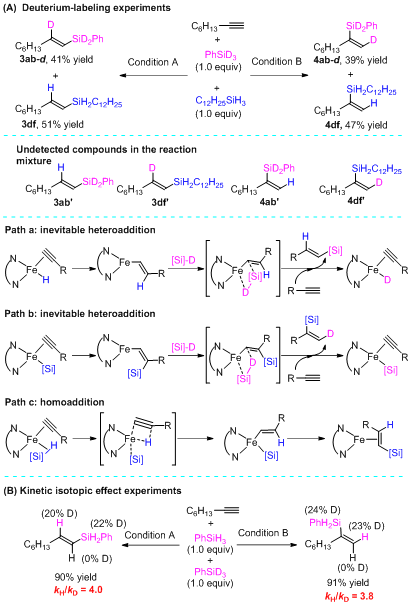
Figure 3. Isotope labeling and dynamic isotope experiments
Further DFT calculation showed that, the energy of iron-catalyzed hydrogen transfer from silane to alkyne (as ligand to ligand) in triplet state is the lowest (Figure 4a). In the process of hydrogen transfer from silane to alkyne promoted by iron-based catalysts, the aryl substituents of the phenanthroline ligands can affect the degree of orbital overlap between substrates and catalysts, thus realizing the regulation of reaction regioselectivity.
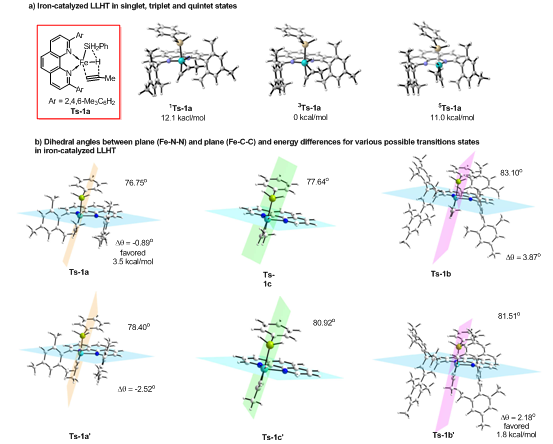
Figure 4. Mechanism of regioselective regulation
In a word, the Zhu group’s latest research expands the application range of iron-based catalysts, shows the potential of iron-catalyzed reactions, provides an effective method for the synthesis of novel organosilicon reagents, and deepens the understanding of the mechanism of iron-catalyzed reactions, which is of great significance for the development and the exploration of unique catalytic performance of open-shell catalysts.
The related results were published in J. Am. Chem. Soc. (https://doi.org/10.1021/jacs.0c09083). The first author is Dr. Mengyang Hu.

