Xiaochen Wang’s group’s research result about Asymmetric Hydrogenation of Quinoline Catalyzed by Chiral Spiro Bicyclic Bisboranes was published in Angew. Chem. Int. Ed.
As an important unit of structural scaffolds, tetrahydroquinoline is prevalent in natural products and pharmaceutical molecules, and asymmetric hydrogenation of quinolines is the most convenient method for the preparation of chiral tetrahydroquinoline. Methods that use transition metal catalysts with ruthenium and osmium as active centers have been extensively investigated, and some of them exhibit high enantioselectivity. However, the high cost and low turnover numbers (TON) limit their applications. More importantly, because of the reactivity of unsaturated functional groups, such as olefins, alkynes, and electron-rich heteroarenes, under metal-catalyzed hydrogenation conditions, it’s hard to preserve these groups during the hydrogenation of the quinoline. Indeed, most existing methods reported are only based on non-functionalized quinoline substrates.
In order to solve these problems, Xiaochen Wang’s group developed a series of chiral spiro bicyclic bisboranes catalyst, based on the previous research on chiral bicyclic bisboranes catalysts (Angew. Chem. Int. Ed. 2018, 57, 15096–15100). The catalysts are synthesized in situ by means of hydroboration reactions of C2-symmetric spiro bicyclic dienes with HB(C6F5)2 and HB(p-C6F4H)2, and gives excellent reactivity (TON up to 460) and enantioselectivity (up to 99% ee). Moreover, this series of catalysts contains attractive features of broad functional group tolerance, which can preserve some unsaturated functional group such as olefins and alkynes. Among them, the turnover numbers of 460 is the highest record of the known chiral boron-catalyzed hydrogenation reaction.
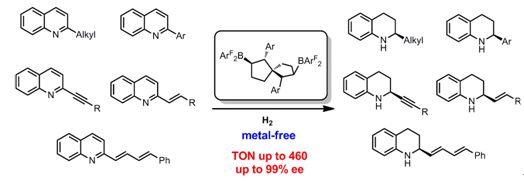
This work was recently published in Angew. Chem. Int. Ed. 10.1002/anie. 201900907. Master graduate ******** and third-year master's student Junjie Tian are co-first authors of the paper.
Full text url: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201900907
The research received financial support from the 1000-Talent Youth Program, the National Natural Science Foundation of China (21602114 and 21871147), the Natural Science Foundation of Tianjin (16JCYBJC42500), and the State Key Laboratory of Element-organic Chemistry (Nankai University). Dedicated to the 100th anniversary of Nankai University!

