The chiral control mechanism of aryl iodide-catalyzed asymmetric conversion of olefins is deciphered by Nankai, Harvard and UCLA’s joint team
Chiral control model for high-valence iodine catalyzed olefin bifunctionalization with C2 axis symmetry
Nankai News Network (Reporter Ma Chao) Recently, Academician Cheng Jinpei and Associate Professor Xue Xiaosong from Nankai University collaborated with Prof. Hawke of the University of California, Los Angeles, and Professor Eric Jacobson of Harvard University to catalyze olefin asymmetry in high-valence iodine achieved significant breakthrough in illuminating the mechanism of chiral geminal difluorination reaction catalyzed by aryl iodide. The research results were published in Journal of the American Chemical Society. Hawke and Xue Xiaosong are co-authors.
The fluorine element is called the “the little giant” in the periodic table. The introduction of a fluorine atom or a fluorine-containing group into an organic compound tends to dramatically change its physical, chemical, and physiological properties. Therefore, fluorine-containing organic compounds are widely applied in the fields of medicines, pesticides, and materials. Among the many fluorine-containing groups, difluoromethyl groups are generally considered to be bioisosteres of hydroxyl groups and mercapto groups. The introduction of such groups into drug molecules generally improves their metabolic stability and oral bioavailability. .
A series of problems caused by the clinical use of racemic drugs, such as drug efficacy, drug transport, metabolism, toxicity, etc., have attracted more and more attention. For the synthesis of drug molecules, the development of chiral catalytic synthesis of difluoromethyl-containing functional groups is of great significance. Xue Xiaosong introduced.
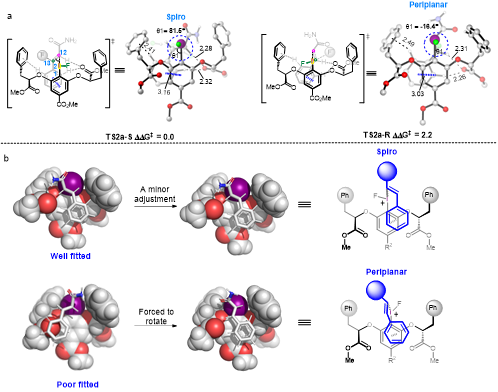
In 2016, the Eric Jacobson team used the chiral aryl iodide catalytic strategy to efficiently and selectively to construct a quaternary carbon chiral center containing difluoromethyl groups under mild conditions. However, how does chiral aryl iodide catalyze this reaction and how can it result in such high selectivity? These two key issues remain unsolved. High-valence iodine has similar reaction properties to transition metals, and its non-toxic safety and economical cost are in line with the development trend of modern chemistry. However, the high-valence iodine catalyzed asymmetric transformation has always been one of the most challenging research topics in the field of high-valence iodine chemistry. The key problem that restricts the development of this field is the ambiguity of the relevant reaction mechanism and the lack of understanding of the chiral control mechanism.
In 2016, Academician Cheng guided us to study the mechanism of geminal difluoride of styrene derivatives mediated by high-valence iodine reagents through DFT calculation, and we found a new activation model of the reagent. Xue said. On the basis of previous research, the research team cooperated with Prof. Hawke and Professor Eric Jacobson to investigate the mechanism of asymmetric geminal fluorination of styrene derivatives by chiral aryl iodide, conducting in-depth theoretical research in the chiral control mechanism. “We examined the mechanism of the reaction in detail, including various possible activation mechanisms of the catalyst, how the difluoromethyl functional group formed, and finally proposed a chiral control model suitable for understanding and predicting C2 axis symmetry of high-valence iodine catalyzing olefin bifunctionalization reaction.
In this model, the chiral pocket of the catalyst enables the recognition of the substrate and the Latent chiral surface, which not only explains and predicts the substituent effects of the catalyst and substrate observed in this experiment, but also provide a theoretical basis for the rational design of high-valence iodine catalyzed asymmetric conversion of olefins. The study will also deepen people's understanding of the chemical reactivity of high-valence iodine helping develop greener and cleaner chemistry based on high-valence iodine.
It is understood that Journal of the American Chemical Society published this achievement for the 70th birthday of Academician Cheng Jinpei.
The research was funded by the National Natural Science Foundation of China and Nankai University.

