Research fellow Fujun Li’s group reported bismuth used as anode for potassium-ion batteries
Potassium-ion batteries (KIBs) are an emerging, promising battery technology for large-scale electric energy storage, because of the abundant potassium resources and redox potential of K+/K close to Li+/Li (-2.93 V vs.-3.04 V). These merit KIBs with potentially low cost and high energy density. The KIBs are based on the extraction/insertion of K+ from/into electrodes. However, the structure stability of electrode materials is plagued by the large-sized K+ (2.76 Å in size). How to build a KIB with high electrochemical performance and avoid K dendrite formation is faced by materials challenge, which is strongly dependent on anodes. Graphite has led to huge success of Li-ion batteries, but its accommodation for the K+ counterpart needs improvement. Suitable anodes for reversible K storage are still lacking.
The redox potential of anode determines the potassiation/depotassiation process and has big effect on the output voltage of full KIBs. It should be higher by ~0.3 V or more than the deposition potential of K to avoid dendrite formation for safety concern, and lower than 1.5 V not to compromise the voltage of full KIBs. The classical conversion reaction has been revealed to result in large discharge/charge voltage hysteresis and instability in metal-ion batteries due to its intrinsic multicomponent reaction.
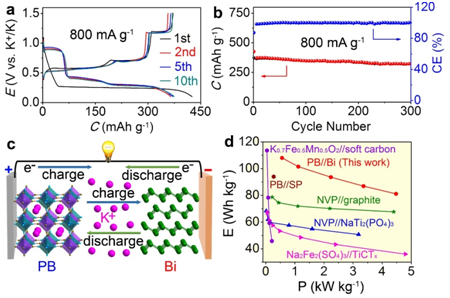
Based on the research background, research fellow Fujun Li’s group constructed a full KIB with commercial Bi as anode, 1.0 M of KPF6 in dimethoxyethane(DME) as electrolyte, and Prussian blue K0.72Fe[Fe(CN)6] as cathode. The full KIB exhibited high capacity retention of 86.5% after 350 cycles and high energy density of 108.1 Wh kg-1 at power density of 566 W kg-1. These are favored by the unique K storage of Bi in the DME-based electrolyte. It is developed into a 3D porous integrity during electrode reactions with three two-phase reactions of Bi↔KBi2, KBi2↔K3Bi2, and K3Bi2↔K3Bi at flat plateaus of safe potentials between 0.3 and 1.15 V. They are related to the strong chemical adsorption of DME molecules on Bi, as confirmed by DFT calculation. Such excellent performance of the full KIB will encourage more investigations into this new battery technology and merit the promising application in large-scale electric energy storage.
The excellent electrochemical performance of the full KIB promises this emerging battery technology potential application in large-scale electric energy storage, and will attract more academic and industrial research interests in the near future.
This work was supported by the Ministry of Science and Technology of China, and National Natural Science Foundation of China. The first author of this research is the PhD candidate Kaixiang Lei.
More detailed information, please click here:
http://onlinelibrary.wiley.com/doi/10.1002/ange.201801389/full

