Work of Zhenhua Yan, Fangyi Cheng, and Jun Chen’s Group Published in Angew: Regulating Electrocatalytic Oxygen Reduction Activity of a Metal Coordination Polymer via d–π Conjugation
Oxygen reduction reaction(ORR) is considered to be the key reaction in fuel battery and metal-air battery. Due to various problems in multi-step electron transfer process such as kinetic slowness and high overpotential of the reaction, Pt/C-based catalyst is usually necessary to lower the overpotential and speed up the reaction rate. However, use of noble metal Pt restricts the development and practical application of relevant instruments on account of its high cost and poor storage. As a result, the development of efficient alternatives to Pt-based electrocatalysts becomes an imperative problem. Non-noble transition metal (TM) complexes have attracted growing interest due to their low cost, high elemental abundance, well-defined active sites and considerable performance. Yet problems like low catalytic activity, insufficient stability, obscurity of coordination structure and the relationship between coordination structure and catalytic activity of ORR remain to impede further development of such catalysts.
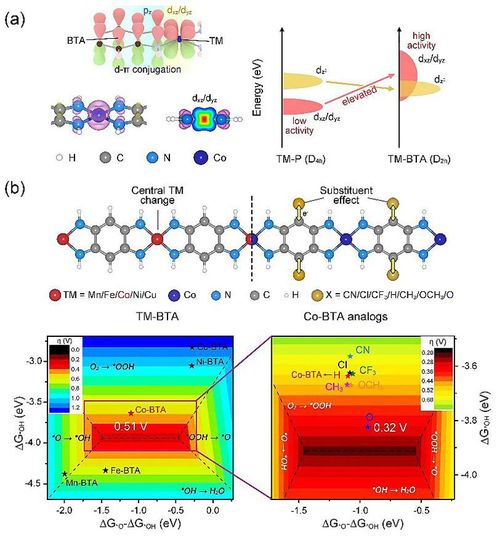
Figure 1.(a) d-π conjugation effects of transition metal complexes, (b) Design of
d-πconjugated complexes and theoretical prediction on the ORR activity volcano.
Given the aforementioned current situation and researching problems, Professor Zhenhua Yan, Professor Fangyi Cheng and Academician Jun Chen from Nankai University cooperated to designed a series of well-structured d-π conjugated linear complexes with uniform metal activity sites, namely TM-BTA(TM=Mn/Fe/Co/Ni/Cu,BTA=1,2,4,5-benzenetetramine) coordination polymers as template catalysts, and realized theoretical simulation of electrocatalytic oxygen reduction is in good agreement with the experimental results. The strong d-π conjugation in these complexes elevates the dxz/dyz orbitals of TM centers to enhance intermediate adsorption and strengthens the electronic modulation effect from substitute groups on ligands(Fig.1a). Based on these facts, researchers proposed a two-step strategy to optimize the ORR activity of TM-BTA analogs by screening preliminarily central TM ions and subsequently substituent groups. Via systematic DFT calculation, researchers provided an ORR activity volcano corresponding to different central metal atom and ligand substituents, which indicates that oxygen-modified Co-BTA (Co-TABQ, TABQ=tetramino-benzoquinone) is the most catalytically active as a result of optimized adsorption strength for intermediates and demostrates best ORR activity due to its highest proximity to the top of the volcano(Fig. 1b).
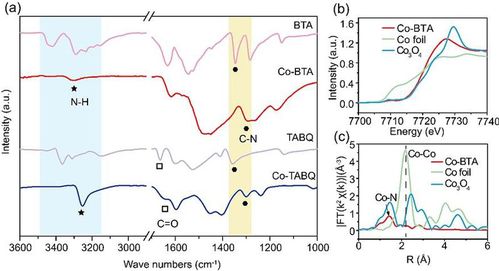
Figure 2. Structural characterization of Co-BTA-based catalyst.
(a) FT-IR graph. (b)XANES spectrum. (c) EXAFS spectrum.
Co、Ni、Cu-BTA and Co-TABQ were successfully prepared in the experiment under facile ambient condition. As for Co-BTA, Fourier-transform infrared (FTIR) spectrum (Figure2a) displays a single peak around 3300cm-1, which corresponds to N-H stretching vibration of -NH in secondary amines. From the extended X-ray absorption fine structure (EXAFS) spectra (Figure2c), the coordination number derived from EXAFS fitting is 3.5±0.9, which further supported the successful synthesis of Co-BTA. From the ORR polarization curves (Figure3a), Co-BTA/C exhibits an E1/2 of 0.77V, which is 40 and 90mV higher than those of Ni-BTA/C and Cu-BTA/C, respectively. As shown in Figure 3b, Co-BTA and Cu-BTA exhibits remarkable ORR activity, while four and two-electron reactions occur simultaneously when it comes to Ni-BTA. The sequence of three complexes correspond with theoretical calculation.
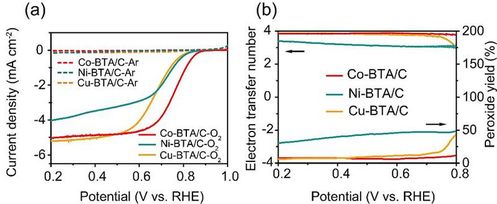
Figure 3. (a) LSV graph and (b) electron transfer number and
selectivity in peroxide of TM-BTA-based catalyst in 0.1M KOH
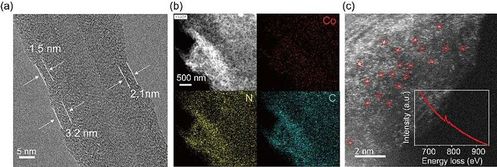
Figure 4. (a) TEM picture (b) Mapping picture and (c) HAADF-STEM
picture of Co-BTA@CNTs, illustration provided by EELS.
To further improve the conductivity, Co-BTA was in situ grown on CNTs to form Co-BTA@CNTs. By taking advantage of the π-π interaction, an amorphous layer (thickness of 1–4nm) of Co-BTA is coated on CNTs(Fig. 4a). Elemental mapping reveals homogeneous distribution of Co, N, and C in Co-BTA@CNTs (Figure4c). High-angle annular dark-filed scanning TEM (HAADF-STEM) image shows a high density of atomically isolated bright spots. Eectrochemical test showd that the half-wave potential of Co-BTA@CNTs increased to 0.83 V(Fig. 5a).
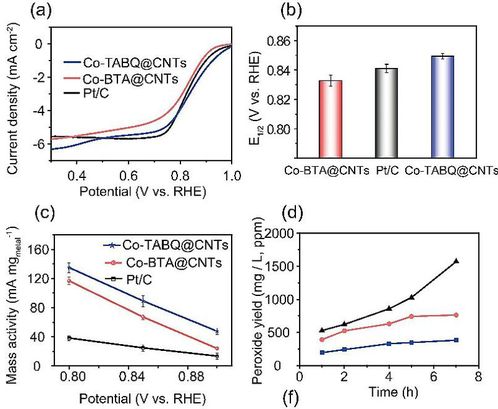
Figure 5. (a)Voltammetry of Co-BTA@CNTs, Co-TABQ@CNTs and Pt/C. (b) Comparison of mass activity of electrocatalysts at various potentials. (c)Comparison of mass activity of electrocatalysts at various potentials. (d) Accumulation of H2O2 over time under a constant current density of 40mAcm-2.
Previous calculation suggests that inducing oxygen substituent to the ligand is a efficient way to improve catalyzing activity, and corresponding synthesis was also carried out experimentally. Figure 5a-b shows that Co-BTA@CNTs exhibits remarkable ORR activity with Eonset of 0.95V, E1/2 of 0.83V and Tafel slope of 65mVdec-1, comparable to commercial Pt/C (0.99V, 0.84V and 59mVdec-1). In addition, Co-TABQ@CNTs significantly surpasses Pt/C in metal-based specific activity (current normalized by metal mass), attaining a high value of 127mAmgmetal−1 at 0.8V. The Co-BTA@CNTs and Co-TABQ@CNTs catalysts were also tested at a high current density of 40mAcm-2 using gas diffusion electrode. As depicted in Figure5d, the H2O2 yield on Co-TABQ@CNTs is much lower than that on Co-BTA@CNTs and Pt/C. The rapid rise of peroxide generation on Pt/C after 5h indicates undesirable activity degradation, which can be ascribed to the apparent agglomeration of platinum nanoparticles after catalytic testing. Thus, Co-TABQ@CNTs and Co-BTA@CNTs outperform Pt/C in terms of stability and selectivity during the ORR electrocatalysis at a high current density.
The experimental result and theoretical simulation exhibited great consistency by virtue of Well defined active center. This work provides mechanistic insight of the d–π conjugation effect on ORR, and enlightens rational searching TM complexes as high-performance electrocatalysts.
This work was published in Angew. Chem. Int. Ed., and the first authors were Youxuan Ni and Liu Lin.This work was supported by the National Key Research and Development Program, the Key Project of the National Natural Science Foundation of China, the General Project and the Youth Fund.

Regulating Electrocatalytic Oxygen Reduction Activity of Metal Coordination Polymer via d-π Conjugation
Youxuan Ni,# Liu Lin,# Yuxin Shang, Lin Luo, Liubin Wang, Yong Lu, Yixin Li, Zhenhua Yan,* Kai Zhang, Fangyi Cheng,* Jun Chen*
Angew. Chem. Int. Ed., 2021, DOI: 10.1002/anie.202104494

