Lehui Xiao’s Group Nano Res: Curved carbon photo-oxygenation catalysts for the suppression and nanoscopic imaging of β-amyloid peptides fibrillation
From NanoResearch
Background
Alzheimer's disease(AD) is the most common chronic neurodegenerative disease among elderly group, which early shows in remembering difficulty. As the disease advances, symptoms can include problems with language, mood swings, movement disorder, impairment in visual spatial cognition and even behavioral or personality issues-- interferes daily life and declines bodily functions, ultimately leading to death. According to WHO, in modern society AD has been the fourth major killer after cardiovascular disease, cancer and stroke. However, there are only 5 AD drugs on neurotransmitters currently approved by the FDA that could only relieve the symptoms but cannot improve the situation of cranial nerve damage. Thus, during treatment it is biomedically significant to suppress aggregation of β-amyloid(Aβ) and trigger disaggregation of mature fibrils. Also, visualizing Aβ aggregates at high spatial resolution is beneficial for assessing the peptide assembly kinetics.
Introduction
Prof. Lehui Xiao(Nankai University), Prof. Yanjun Zhao(Tianjin University), Prof. Haibin Luo(Sun Yat-sen University) conjugated corannulene (Cor) with rhodamine B isothiocyanate (Rhb), designed a new molecule namely Cor-Rhb (Figs. 1(a)). Unlike conventional fluorophores, Cor-Rhb shows spontaneous blinking with low duty cycle, high photon output and sufficient “on-off” switching cycles, which is crucial to obtain nanoscopic images under physiological conditions. Using Cor-Rhb, we explore the morphology dependent toxic effect of Aβ1–40 aggregates towards PC12 cells and demonstrate that the most toxic form of Aβ1–40 aggregates is the short fibrils with length shorter than the optical diffraction limit (Figs. 1(b)). In addition, the efficient inhibition of Cor-Rhb on Aβ1–40 assembly is confirmed with microscopic characterizations. We find that Cor-Rhb interacts directly with mature fibrils, triggering their disaggregation under light illumination (Figs. 1(c)). Collectively, Cor-Rhb provides the potential as a multifunctional therapeutic agent against amyloid-related diseases and an imaging probe for super-resolution based biological applications.
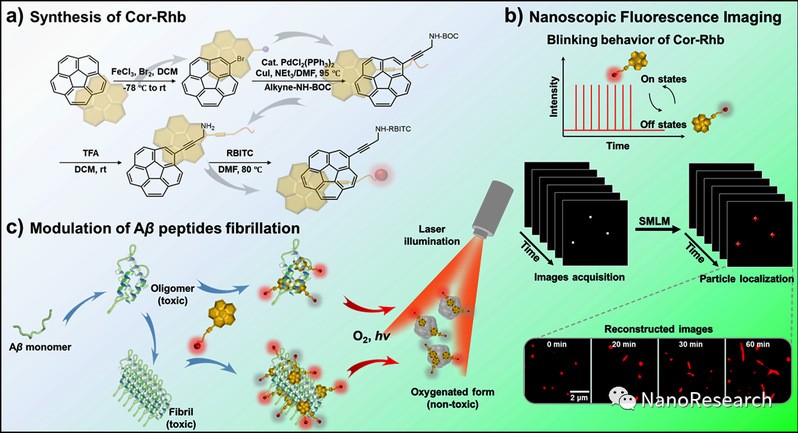
Figure 1 (a) Schematic illustration of the procedures to fabricate Cor-Rhb. (b) Nanoscopic imaging of amyloid fibrils with Cor-Rhb via SMLM. (c) The method to modulate Aβ1–40 fibrillation with Cor-Rhb under laser illumination.
Discussion
Research finds that Cor-Rhb possesses improved blinking behavior, exhibiting long “off” state in the blinking process, which is well-suited for single-molecule localization imaging. The fibrils stained with Cor-Rhb reveal a comparable fluorescence image to those stained with the commercial ThT. According to the reconstructed nanoscopic fluorescence image, the measured diameter of the fibril is 24 ± 5 nm, which is close to the actual size of Aβ1–40 fibrils. The resolution is improved by more than ten-fold in contrast to that of the epi-fluorescence image.
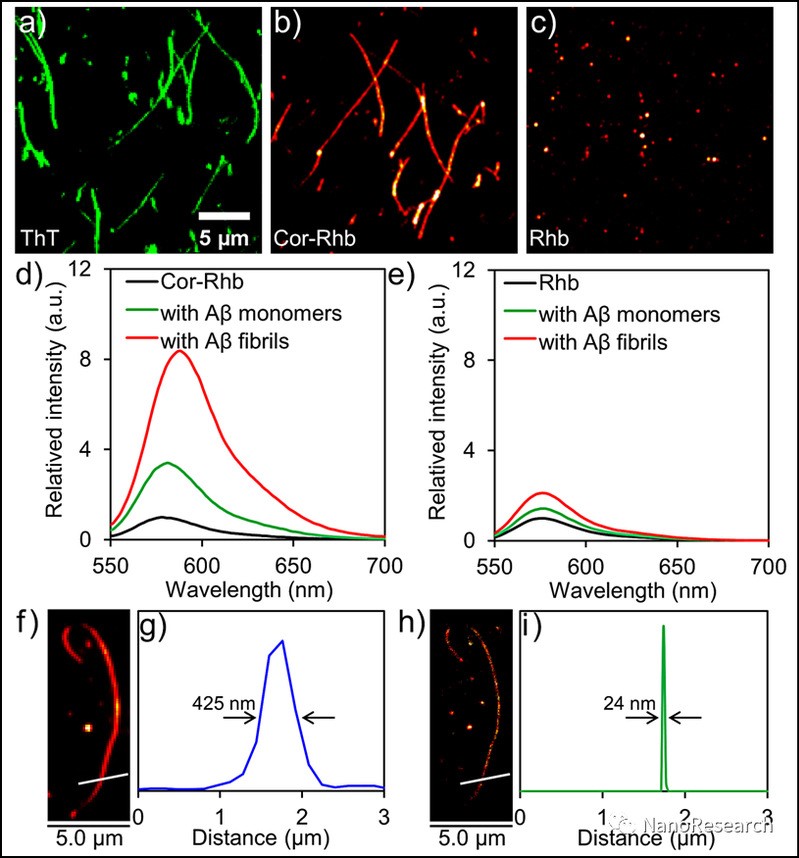
Figure 2 (a)–(c) Epi-fluorescence images of preformed Aβ1–40 fibrils stained by ThT (a), Cor-Rhb (b) and Rhb (c), respectively. (d) and (e) Fluorescence spectra of Cor-Rhb (d) and Rhb (e) in the absence and presence of Aβ1–40 monomers and fibrils. (f) and (h) Representative epi-fluorescence image (f) and the corresponding reconstructed nanoscopic fluorescence image (h) of preformed Aβ1–40 fibrils stained by Cor-Rhb. (g) and (i) The corresponding intensity profiles of the white lines in (f) and (h), respectively.
The fibrosis process of Aβ1-40 followed a typical nucleation growth mechanism, including three stages: lag, rapid growth and equilibrium. Through staining the sample with ThT, the inhibition effect of Cor-Rhb was confirmed with fluorescence imaging: Cor-Rhb under laser irradiation generates more ROS than Cor. Because the aggregation of peptides is closely dependent on the intermolecular hydrophobic interactions, oxygenation of hydrophilic oxygen atoms to the peptide or fibril could decrease the aggregative property by stabilizing the hydrated states. Oxidation of Aβ1–40 peptide with Cor-Rhb under laser irradiation can efficiently suppress the aggregation of monomeric peptides and trigger the disaggregation of mature fibrils.
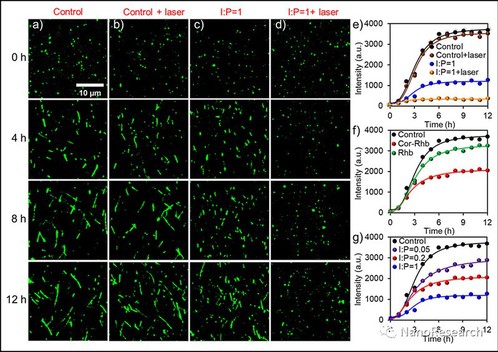
Figure 3 (a) and (b) Fluorescence microscopic characterizations of Aβ1–40 fibrillation process without (a) and with (a) laser irradiation at 0, 4, 8 and 12 h, respectively. (c) and (d) Fluorescence microscopic characterizations of the inhibition effect of Cor-Rhb (I:P = 1) on Aβ1–40 fibrillation process without (c) and with (d) laser irradiation at 0, 4, 8 and 12 h, respectively. (e) The time dependent ThT fluorescence intensity measurements of Aβ1–40 mixed with Cor-Rhb (I:P = 1) on the effect of laser irradiation. (f) The effect of Cor-Rhb and Rhb at I:P = 0.2 on Aβ1–40 fibrillation process. (g) The dosage dependent inhibition effect of Cor-Rhb (I:P = 0.05, 0.2 and 1, respectively) on Aβ1–40 fibrillation process.
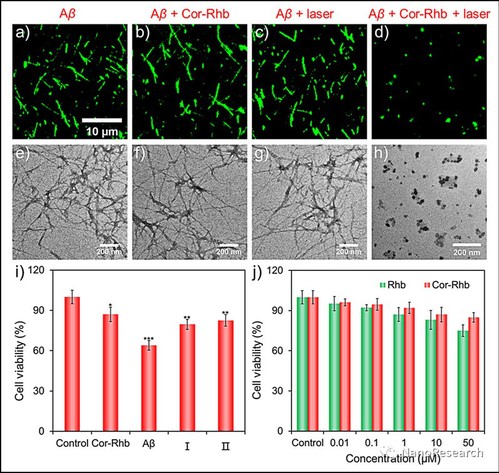
Figure 4 Fluorescence microscopic characterizations of (a) Aβ1–40 fibrils, (b) the depolymerization effect treated with Cor-Rhb (I:P = 1) for 12 h without laser irradiation, (c) the depolymerization effect under pure laser irradiation for 30 min and further incubated for 12 h, and (d) Aβ1–40 fibrils treated with Cor-Rhb (I:P = 1) under laser irradiation for 30 min and then further incubated for 12 h. (e)–(h) The corresponding TEM images from the results as shown in (a)–(d) respectively. (i) Relative cell viability of PC12 cells after being treated with Cor-Rhb (50 μM), Aβ1–40 fibrils (50 μM), monomeric Aβ1–40 peptides mixed with Cor-Rhb (I:P = 1) after laser irradiation for 30 min (I) and Aβ1–40 fibrils treated with Cor-Rhb (I:P = 1) after laser irradiation for 30 min (II) for 24 h. (j) Relative cell viability of PC12 cells after being treated with different concentrations of Rhb, Cor-Rhb (0.01, 0.1, 1, 10, and 50 μM) for 24 h.
For a better understanding of the structural characteristics of the interaction between Cor-Rhb and Aβ1–40 peptide, submicrosecond-scale molecular dynamics (MD) simulations were processed for the complex of monomeric Aβ1–40 and Cor-Rhb. It is convinced that the dominant driving force for stabilization when Aβ1–40 binds with Cor-Rhb is ascribed to both the electrostatic and van der Waals interaction from the time-dependent interaction energy curves. In order to thoroughly analyze the contribution of each residue to the interaction between Cor-Rhb and Aβ1–40, energy decomposition for the binding free energies was additionally processed with the MM-GBSA method. Results indicates that Cor-Rhb makes strong interaction with Aβ1–40 mainly by π–cation/π–π interactions with Arg5/Ph
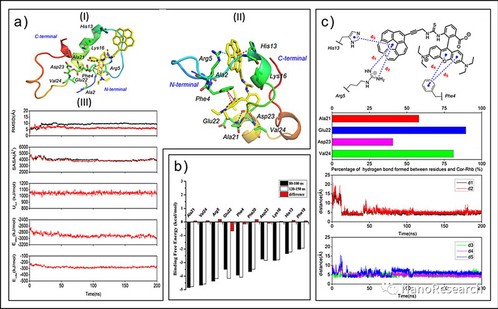
Figure 5 MD simulation studies for monomeric Aβ1–40 bound with Cor-Rhb. (a) Molecular docking pattern of Cor-Rhb with Aβ1–40 (PDB ID: 2LFM, depicted in solid ribbon) (I), and stable MD binding pattern of Cor-Rhb with Aβ1–40 after 60 ns in the binding site (II). Hydrogen bond, π–π and π–cation interactions are depicted in blue, red and green dashed lines, respectively. (III) Time-dependent plots for RMSD of atomic positions, SASA for the Aβ1–40 monomer and the Aβ1–40 monomer with Cor-Rhb, Utot, Eelec and Evdw for the Aβ1–40 monomer with Cor-Rhb, respectively (from the top). The curves of Aβ1–40 monomer and Aβ1–40 monomer with Cor-Rhb are depicted in black and red, respectively. (b) Decomposition of the key residue contributions to the binding free energies for the complex of Aβ1–40 with Cor-Rhb. (c) Hydrogen bond analysis, π–π/π–cation interaction analysis and corresponding distance monitoring for monomeric Aβ1–40 bound with Cor-Rhb for the whole trajectory during MD simulations, respectively (from the top)
About the Author
Prof. Dr. Lehui Xiao, investigator, PhD tutor, college of Chemistry, Nankai University. Prof. Xiao works on recognition, motion detection and space-time fluctuations at single molecule/nanoparticle scale. In his group, single nanoparticle is applied as the probe for the detection of biomolecules in solution or within living cells with single particle spectroscopy. They are also developing some robust optical imaging methods for the dynamic tracking of individual plasmonic particles on the cell membrane or inside living cells. He has published a total of 70 SCI articles as the first author/corresponding author on J. Am. Chem. Soc.(2), Angew. Chem. Int. Ed.(4), ACS Nano(3), CCS Chem.(2), Chem. Sci.(3), Anal. Chem.(20). Prof. Xiao is also a youth editor of Chinese Chemical Letters(CCL).
Group website: https://www.xiaolhlab.cn/
Article Information
Yuanyuan Ma, Zhongju Ye, Chen Zhang, Yanjun Zhao*, Hai-bin Luo* & Lehui Xiao*. Curved carbon photo-oxygenation catalysts for the suppression and nanoscopic imaging of β-amyloid peptides fibrillation.Nano Research
https://doi.org/10.1007/s12274-021-3927-5.

