NKU RESEARCHERS REVEAL A NEW MECHANISM OF ORGANIC PHOTOCHEMICAL REACTION
According to a recent paper published in JACS, Nankai University, SIOC at UCAS, and Beijing Normal University collaboratively developed a solid difluorocarbene precursor, difluoromethane bis(sulfonium ylide), with a symmetrical structure of E-CF2-E. This article reports that a new mechanism of organic photochemical reactions was discovered by this team, in which difluorocarbene is generated by spin-forbidden excitation under visible light irradiation.
The project was led by Prof. Liu Tianfei of the State Key Laboratory of Elemento-Organic Chemistry at Nankai University, who is the first corresponding author. Prof. Shen Qilong from Shanghai Institute of Organic Chemistry at UCAS, and Prof. Cui Ganglong from Beijing Normal University are the other two co-corresponding authors. In this article, Liu Shan from Nankai University and Pan Guang-Ning from Beijing Normal University are the co-first authors. Other members of the research team include Dr Ling Yijing and Gao Feng from dsSIOC, as well as Assoc. Prof. Yang Yin from SKLEOC at Nankai University.
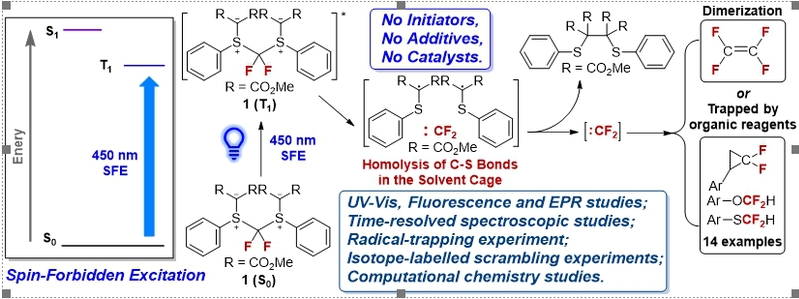
Figure 1. Difluorocarbene Generation via a Spin-Forbidden Excitation under Visible Light Irradiation.
It is important to note that difluorocarbene is a reaction intermediate that exhibits electrophilic properties. It is mainly used in medicine, agrochemistry, and material chemistry to prepare organofluorine compounds containing difluoromethylene groups. As a result of its high reactivity, difluorocarbene must be generated from difluorocarbene precursors under specific conditions. Nevertheless, traditional difluorocarbene precursors require additives, catalysts, or relatively strong reaction conditions in order to yield difluorocarbene. Prof. Liu Tianfei commented, “A new solid difluorocarbene precursor has been described in our research article, difluoromethane bis(sulfonium ylide), that can be used to generate difluorocarbene intermediates via a newly discovered reaction mechanism based on spin-forbidden excitation under visible light irradiation without initiators, additives, or catalysts.”
The researchers conducted systematic experiments and theoretical studies of this newly discovered reaction mechanism based on spectroscopic measurements, kinetic measurements, and computational analysis. The homolytic cleavage of two S–C bonds in compound 1 under irradiation was confirmed by time-dependent EPR spectroscopic studies of the precursor’s free-radical-capturing reaction, as well as the isolation of the dimer of dimethyl (phenylthiol)malonyl radical. Further studies showed that the homolytic cleavage process occurred asynchronously in the solvent cage based on the isotope-labelled scrambling experiments and DFT calculations.

Figure 2. The team from NKU is conducting kinetic measurements using time-dependent EPR spectroscopy. (From right to left, Ms. Liu Shan, Prof. Liu Tianfei, and Assoc. Prof. Yang Yin)
The majority of organic photochemical reactions that people are familiar with require light to excite the photosensitizer from its S0 state to its S1 state through a spin-allowed transition. One of the most innovative aspects of this study is that it is the first time that an organic compound has been demonstrated to undergo a spin-forbidden excitation under visible light irradiation to directly generate the T1 state, which then triggers subsequent reactions that release difluorocarbene intermediates. “Using spin-forbidden excitation under visible light irradiation to produce difluorocarbene has opened up a new avenue for organic photochemistry research, and will assist in the development of new reagents in the future that utilize spin-forbidden excitation reactions, said Prof. Liu Tianfei.
The National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, the National Key Research and Development Program of China, and the ********* Priority Research Program of the Chinese Academy of Sciences funded this research. This research was also supported by the State Key Laboratory of Elemento-organic Chemistry, College of Chemistry at Nankai University, Haihe Laboratory of Sustainable Chemical Transformations, Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials in Shanghai Institute of Organic Chemistry at the Chinese Academy of Sciences, and Key Laboratory of Theoretical and Computational Photochemistry, Ministry of Education, College of Chemistry at Beijing Normal University.
For original information about this paper, please click the following URL
https://doi.org/10.1021/jacs.4c10939
(by Liu Shan, edited by Li Jing)

