Nankai University Research Team Pioneers Novel Pathway for Broad-Spectrum Anti-Influenza A Therapy
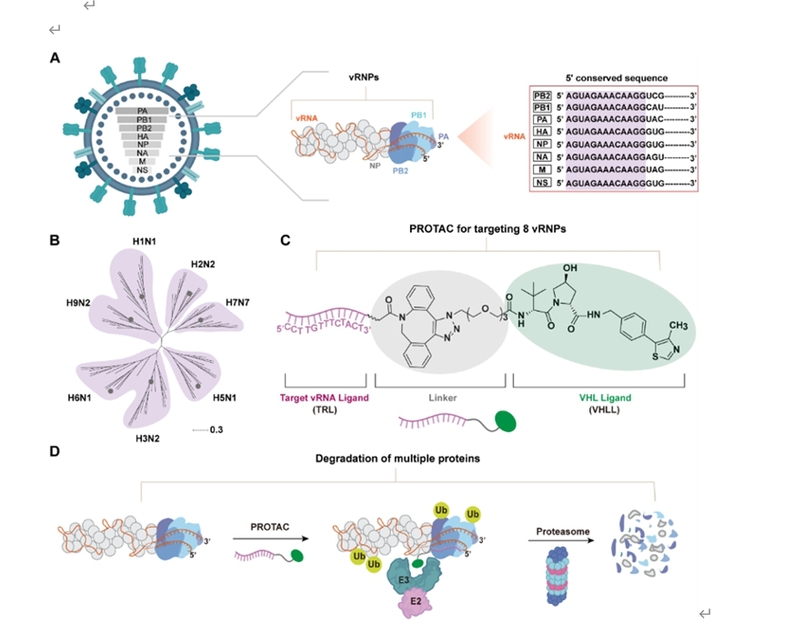
Recently, a research team led by Researcher ********** at the College of Chemistry, Nankai University, achieved a significant breakthrough in broad-spectrum anti-influenza A therapy. Liu’s team developed an innovative multiplexed PROTAC antiviral strategy capable of simultaneously degrading multiple key components of the viral ribonucleoprotein (vRNP) complex, which is the core machinery for viral replication. This study offers a novel solution for potent, durable, and broad-spectrum influenza treatment, which overcomes the limitations of existing therapies. The related findings were recently published in the Journal of the American Chemical Society.
The strategy’s core innovation lies in the ingenious exploitation of a highly conserved “universal code” within the viral RNA—the 5’ untranslated region (5’ UTR). This sequence is remarkably stable across different influenza virus strains and serves as an indispensable “switch” for viral replication. Building on this insight, Liu’s team rationally designed a bifunctional PROTAC molecule. Operating like a precise “Trojan horse,” it can simultaneously perform the tasks of recognition, binding, and targeted degradation. At one end, it features an oligonucleotide ligand that precisely binds to the viral “universal code”; at the other end, it incorporates a moiety responsible for recruiting the cell’s intrinsic protein “shredder”—the proteasome.
These two parts are covalently linked by an optimized linker. Once the PROTAC molecule enters an infected cell, it can simultaneously engage the viral vRNP complex and the cellular proteasome, thereby tagging core viral proteins—including the nucleoprotein (NP) and polymerase subunits PB1, PB2, and PA—for “destruction.” Subsequently, the cell’s proteasome efficiently and thoroughly degrades these crucial components of viral replication, thus dismantling the virus’s ability to replicate at its source.
Schematic Illustration of PROTAC-Mediated Degradation of Multiple Viral Proteins
The antiviral results fully validated the excellent performance of this multiplexed PROTAC strategy. The PROTAC molecule effectively inhibited viral replication in a concentration-dependent manner, with the inhibitory effect lasting for over 48 hours. Its durability and potency significantly outperformed those of single-target control drugs. More importantly, by simultaneously degrading multiple viral proteins, this strategy successfully established a high genetic barrier to resistance. For the virus to develop resistance, it must overcome this hurdle, which requires the concurrent emergence of effective escape mutations across multiple viral protein targets. This constitutes an evasion barrier that is exceedingly difficult for the virus to surmount through natural evolution. The strategy also demonstrated broad-spectrum activity against multiple influenza virus strains, further highlighting its potential as a broad-spectrum anti-influenza therapeutic.
This groundbreaking study pioneers the application of multi-target PROTAC technology in antiviral therapy, representing a paradigm shift from conventional single-target inhibition to a systemic protein degradation strategy for influenza. The work provides a robust conceptual and experimental foundation for the development of next-generation broad-spectrum anti-influenza therapeutics.
(Source: Nankai University News)

